Publications
A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts
X. Huang, Z. Zhao, Y. Chen, E. Zhu, M. Li, X. Duan and Y. Huang
Energy Environ. Sci. 7, 2957-2962 (2014)
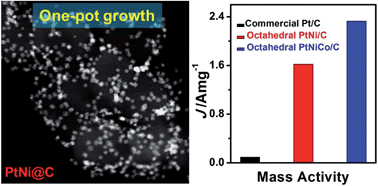
Bimetallic PtNi nanocrystals represent an emerging class of newly discovered electrocatalysts which are expected to exhibit exciting oxygen reduction reaction (ORR) activity. Colloidal syntheses have been proven to be suitable for controlling PtNi nanocrystals with well-defined morphologies and tunable compositions with the use of capping agents or ligands. However, these colloidal PtNi nanocrystals have inherent limitations associated with the ligand-covered surfaces, which not only limit the free access of surface active sites but also hinder electron transport between the catalyst and the support, leading to deteriorated ORR performance. Herein, we report a facile one-pot strategy to synthesize highly dispersive PtNi octahedra directly on various carbon materials without using any bulky capping agents, which enhances the surface exposure of the PtNi octahedra and their catalytic activity over ORR while largely reduces the preparation costs. The obtained octahedral PtNi/C catalysts have high ORR activities of 2.53 mA cm−2 and 1.62 A mgPt−1 at 0.9 V versus RHE, which are far better than those of commercial Pt/C catalysts (0.131 mA cm−2 and 0.092 A mgPt−1, all the ORR measurements were performed at room temperature in O2-purged 0.1 M HClO4 solutions at a sweep rate of 10 mV s−1). This strategy has been extended to fabricate trimetallic PtNiCo octahedra on carbon black with further enhanced activities up to 3.88 mA cm−2 and 2.33 A mgPt−1 at 0.9 V versus RHE. The octahedral PtNiCo/C catalyst is also more stable than the commercial Pt/C under the ORR conditions and shows small activity change after 6000 potential sweeps. The work demonstrates that the carbon-supported Pt-based materials reported herein are promising material candidates with enhanced performances for practical electrocatalytic applications.
UCLA, Department of Chemistry and Biochemistry
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu







