Publications
Autobifunctional Mechanism of Jagged Pt Nanowires for Hydrogen Evolution Kinetics via End-to-End Simulation
Geun Ho Gu, Juhyung Lim, Chengzhang Wan, Tao Cheng, Heting Pu, Sungwon Kim, Juhwan Noh, Changhyeok Choi, Juhwan Kim, William A Goddard III, Xiangfeng Duan, Yousung Jung
J. Am. Chem. Soc. 143, 5355–5363 (2021)
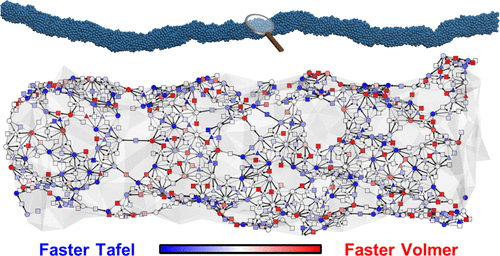
The extraordinary mass activity of jagged Pt nanowires can substantially improve the economics of the hydrogen evolution reaction (HER). However, it is a great challenge to fully unveil the HER kinetics driven by the jagged Pt nanowires with their multiscale morphology. Herein we present an end-to-end framework that combines experiment, machine learning, and multiscale advances of the past decade to elucidate the HER kinetics catalyzed by jagged Pt nanowires under alkaline conditions. The bifunctional catalysis conventionally refers to the synergistic increase in activity by the combination of two different catalysts. We report that monometals, such as jagged Pt nanowires, can exhibit bifunctional characteristics owing to its complex surface morphology, where one site prefers electrochemical proton adsorption and another is responsible for activation, resulting in a 4-fold increase in the activity. We find that the conventional design guideline that the sites with a 0 eV Gibbs free energy of adsorption are optimal for HER does not hold under alkaline conditions, and rather, an energy between −0.2 and 0.0 eV is shown to be optimal. At the reaction temperatures, the high activity arises from low-coordination-number (≤7) Pt atoms exposed by the jagged surface. Our current demonstration raises an emerging prospect to understand highly complex kinetic phenomena on the nanoscale in full by implementing end-to-end multiscale strategies.
UCLA, Department of Chemistry and Biochemistry
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu







