Publications
Organosulfur compounds enable uniform lithium plating and long-term battery cycling stability
Bismark Boateng, Yupei Han, Cheng Zhen, Guangfeng Zeng, Ning Chen, Dongjiang Chen, Chao Feng, Jiecai Han, Jie Xiong, Xiangfeng Duan, Weidong He
Nano Lett. 20, 2594–2601 (2020)
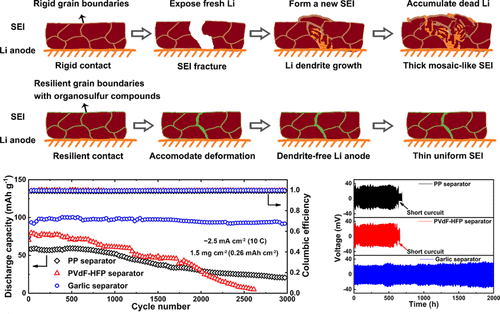
Lithium metal represents an ultimate anode material of lithium batteries for its high energy density. However, its large negative redox potential and reactive nature can trigger electrolyte decomposition and dendrite formation, causing unstable cycling and short circuit of batteries. Herein, we engineer a resilient solid electrolyte interphase on the Li anode by compositing the battery separator with organosulfur compounds and inorganic salts from garlic. These compounds take part in battery reactions to suppress dendrite growth through reversible electrochemistry and attenuate ionic concentration gradient. When the Li anode and the separator are paired with the LiFePO4 cathode, one obtains a battery delivering long-term cycling stability of 3000 cycles, a rate capacity of 100 mAh g–1 at 10 C (2.5 mA cm–2), a Coulombic efficiency of 99.9%, and a low battery polarization. Additionally, with high-loading 20 mg cm–2 LiFePO4 cathodes, an areal capacity of 3.4 mAh cm–2 is achieved at 0.3 C (1 mA cm–2).
UCLA, Department of Chemistry and Biochemistry
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu







