News
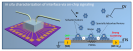
For many sustainable energy technologies, such as fuel cells, batteries, and artificial photo-synthesis, electrocatalysis plays a central role. It has become generally accepted that being able to “see” the electrochemical reactions at molecular level is key to help scientists and engineers better understand, and then achieve major breakthroughs in the development of next-generation energy technologies. This is yet extremely challenging due to the lack of in situ analytical tools (spectroscopic methods that can efficiently access the electrochemical interfaces between solid electrodes and liquid electrolytes while providing selective and accurate signals). A research team led by Prof. Xiangfeng Duan has spent several years working on the development of an overall different tool, using on-chip nano-electronic signaling approach, which is typically applied in semiconductor industry, to help “see” the specific electrochemical interfaces (a process that highly matters to the performance of the materials) while the catalytic process is in action. In this recent study, the UCLA team has used the strategy to visualize a classic interfacial process, anionic adsorptions on platinum surface, and hence identified it as a descriptor for the oxygen reduction kinetics. These findings provide the UCLA team with intriguing fundamental insights into the surface poisoning of reaction kinetics by trace anionic species, and with this efficient way of studying tool, researchers were able to find solutions to increase the poisoning resistance of the fuel cell catalyst (or impurity tolerance) of the fuel cell catalysts, which has been a serious issue for the real-world fuel cell devices (linked to device durability). With many successful applications, the researchers believe such nano-electronic method could further become a general approach to help uncover broader governing principles in the field of electrocatalysis.
607 Charles E. Young Drive East, Box 951569
Los Angeles, CA 90095-1569
E-mail: xduan@chem.ucla.edu







